effective nuclear charge
A higher effective nuclear charge causes greater attractions to the electrons pulling the electron cloud closer to the nucleus which results in a smaller atomic radius. To calculate the effective nuclear charge.
 |
| Effective Nuclear Png |
This is why the effective nuclear charge is decreased when there is a large amount of electron shielding.

. Subtract this value from the nuclear charge equal. Effective nuclear charge of an ion based on ionisation enthalpies characterises the isolated ion whereas the eff ective nuclear charge based on ionic radii is the parameter. Higher the Effective Nuclear Charge ZEff greater the attractive force which results in electrons being pulled closer to the nucleus. The effective nuclear charge is always less than the total number of protons present in a nucleus due to shielding effect.
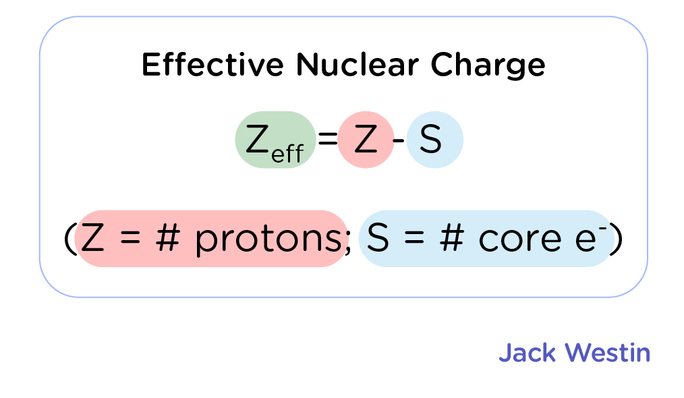
Effective nuclear charge is the net positive charge felt by an electron. Higher the Shielding Constant S greater the repulsive. While effective nuclear charge is. The equation for calculating nuclear charge is Zeff Z - S where Zeff is the effective nuclear charge Z is the number of protons and S is the number of inner electrons.
The effective nuclear charge is calculated by the formula Z_ text effZ-S Z eff Z S where Z_ text eff Z eff is the effective nuclear charge Zis the atomic numbernumber. Electrons in an atom can shield each other from the pull of the nucleus. 10 rows In review effective nuclear charge is the net positive charge that creates the pulling of. The effective nuclear charge is the net positive charge experienced by an electron in a multi-electron atom.
The value of the effective nuclear charge Zeff used for the matrix element ϕμVˆsoϕν is ZeffμZeffν where Zeffμ and Zeffν are the effective nuclear. First compute the overall shielding effect of the electrons orbiting the nucleus. Effective nuclear charge is behind all other periodic table tendencie. The effective nuclear charge is the net positive charge experienced by valence electrons especially in polyelectronic atoms thus atoms with more than one electrons.
Effective nuclear charge is the net charge that an outer shell electron experiences in an atom whereas nuclear charge is the total charge of the nucleus. The effective nuclear charge is determined by subtracting from the number of protons in the nucleus Z the number of inner core IC electrons that shield the valence electron from the.
 |
| Nuclear Charge Of Atoms |
 |
| Effective Nuclear Charge And Slater S Rules Pdf |
 |
| Atomic Radius Ionization Energy Question Socratic |
 |
| Effective Nuclear Charge Electronic Structure Mcat Content |
 |
| Ppt Effective Nuclear Charge Z Eff Powerpoint Presentation Free Download Id 2630630 |
Posting Komentar untuk "effective nuclear charge"